
Our Journey to the US

US Infant Formula Act
To understand Kabrita’s journey to the United States, you have to first understand how infant formula is regulated in our country.
The Center for Food Safety and Applied Nutrition (CFSAN), which is part of the Food and Drug Administration (FDA), regulates all food.
Infant formula is a food, meaning this department also oversees infant formula via the US Infant Formula Act, which is “one of the most specific and detailed acts ever passed by Congress.”68-70
This Act ensures the safety of the most vulnerable population, by outlining strict requirements such as:
- Ingredient safety
- Nutrition thresholds for 30 nutrients
- Quality control measures
- Labeling accuracy
- Manufacturing standards

Extensive FDA review process
To legally sell infant formula in the US, manufacturers have to go through an extensive review process with the FDA to:
1) Ensure that all nutrition requirements are met
2) Tell the FDA where and how the formula will be made
3) Notify the FDA and submit all required documents
4) Verify the formula with the FDA.
Once the FDA has completed the review and determined all requirements are met, a "no further questions" letter is sent to acknowledge that the product can be legally sold in the US.
Kabrita Goat Milk-Based Infant Formula has gone through this extensive process.
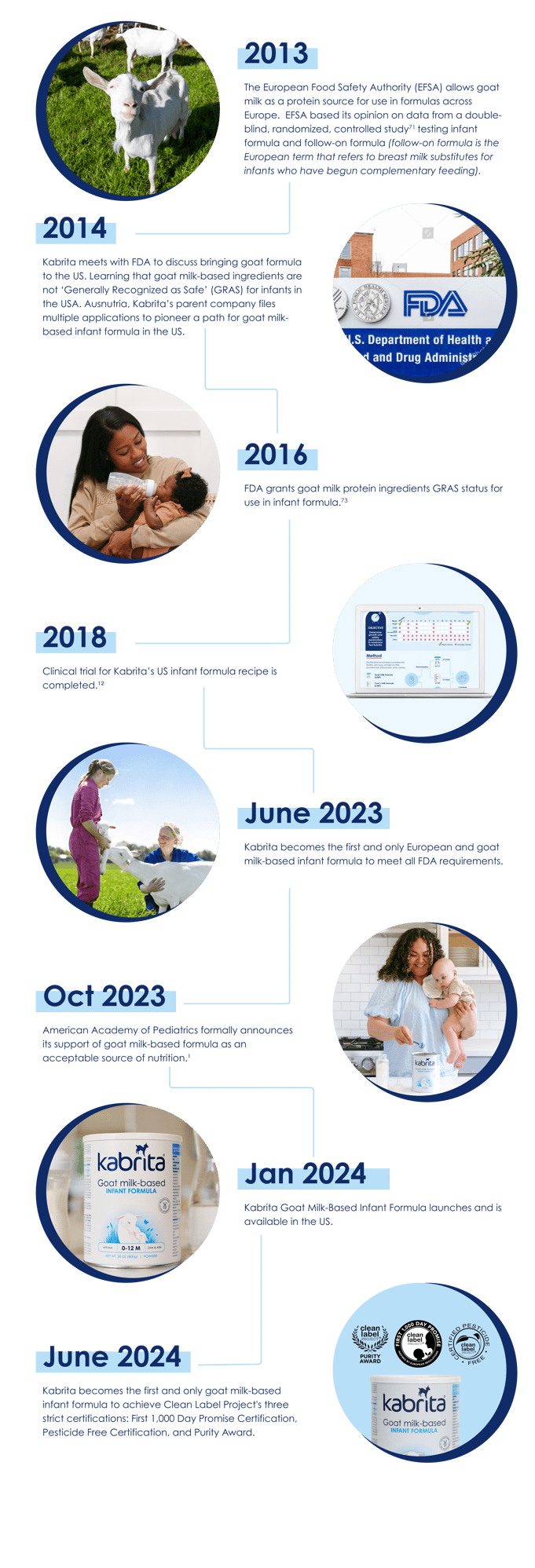
Our Journey to the US
Follow the decade-long journey that Kabrita Goat Milk-Based Infant Formula has taken
to complete the full FDA infant formula review process.
How Kabrita stacks up
to other goat milk-based infant formulas
available to US families
-1.png?width=600&height=432&name=Journey%20to%20the%20US%20page%20Comparison%20Chart%20(1)-1.png)







