
Clinical trial
Industry standard and FDA requirements are to do an infant formula clinical study and share the results with the FDA. We believe in advancing the field of infant nutrition and goat milk at large, so we published our clinical trial for everyone to read.
Goat Milk-Based Infant Formula in Newborns: A Double-Blind Randomized Controlled Trial on Growth and Safety
No time to read the full publication?
Here is the gist...
This randomized, double-blind trial, a growth monitoring study designed to meet the U.S. Food and Drug Administration’s review requirements, aimed to assess the growth and safety of newborns fed with a goat milk-based infant formula.12
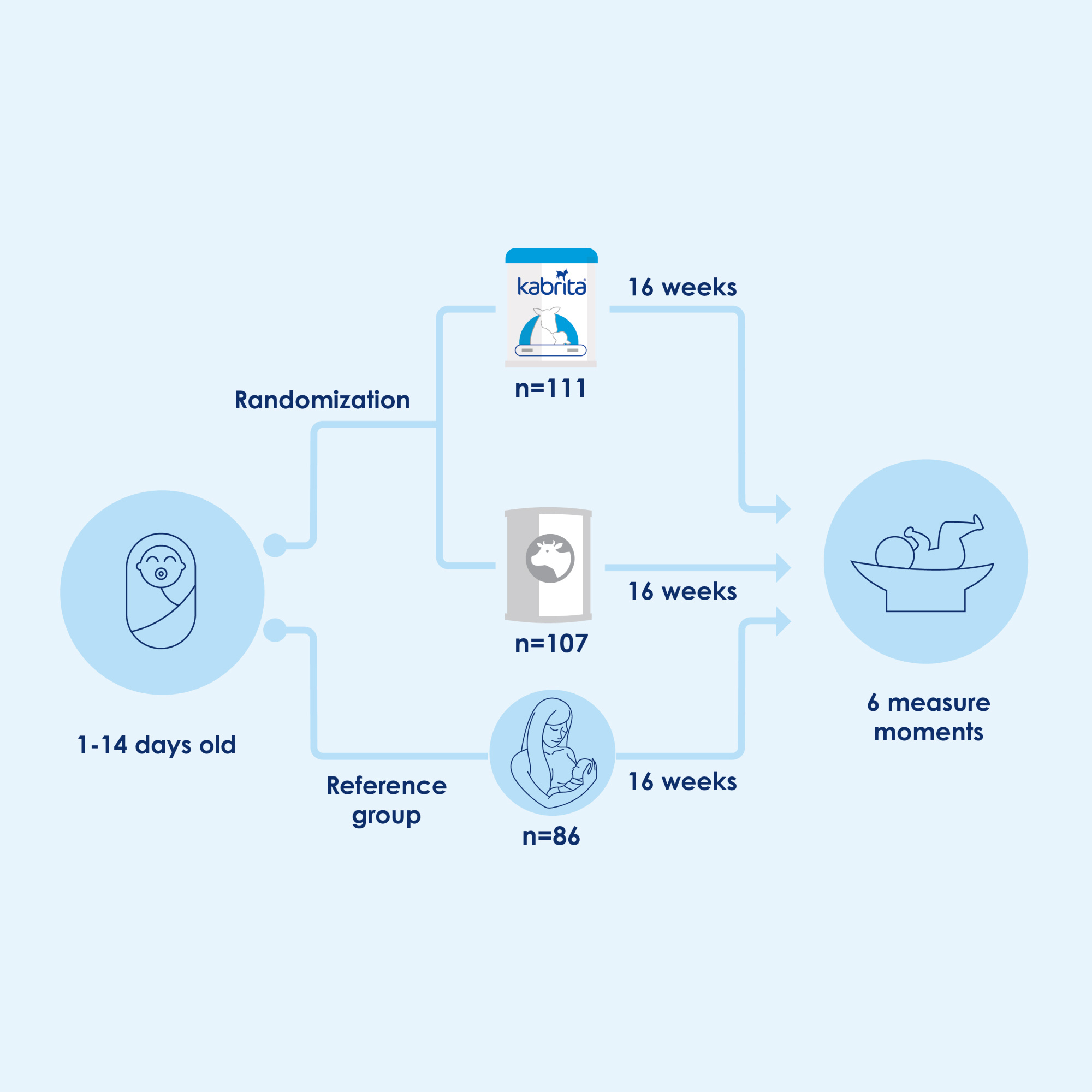
Methods
We compared Kabrita Goat Milk-Based Infant Formula to cow milk-based infant formula and used breast-fed infants as a reference group.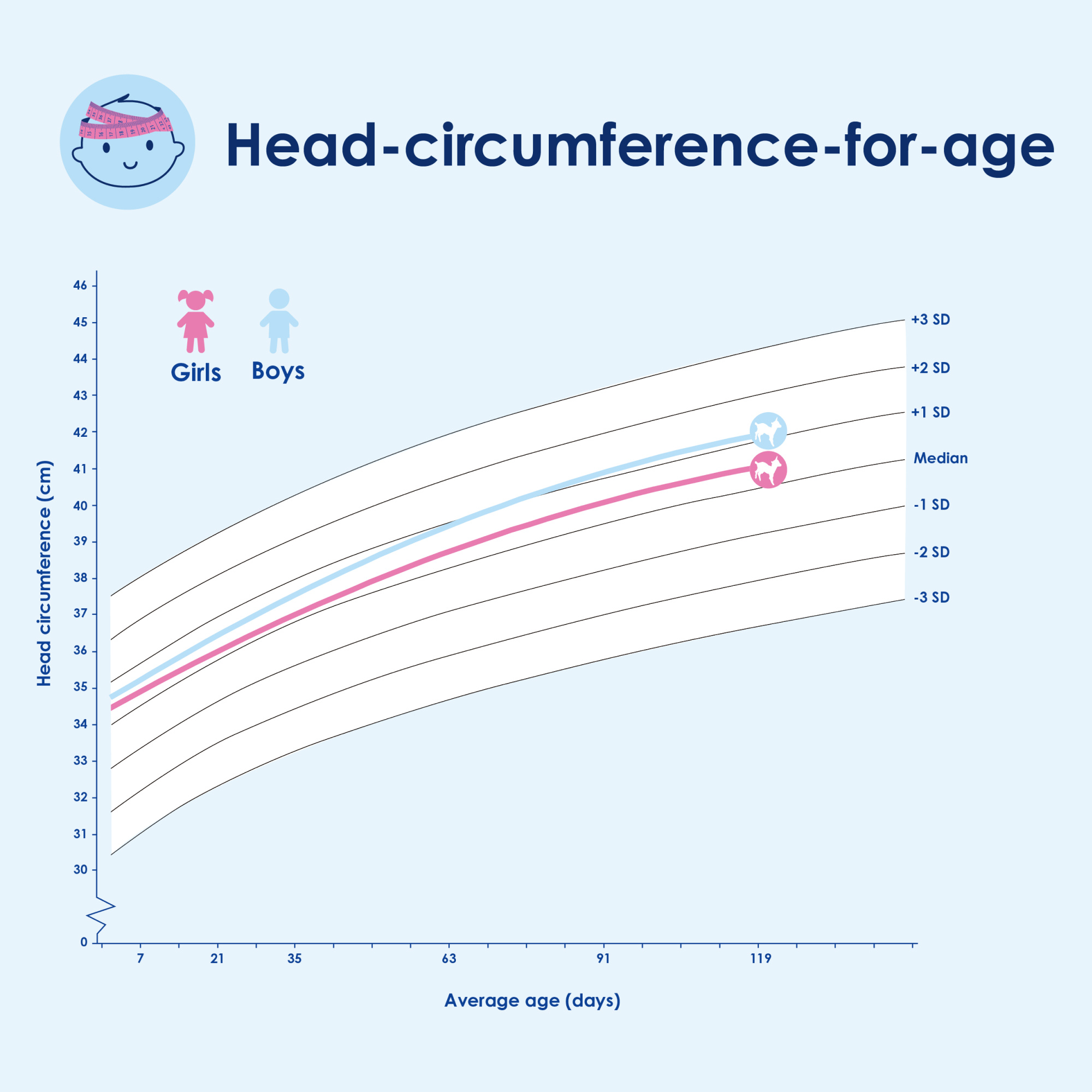
Results
Kabrita Goat Milk-Based Infant Formula showed healthy growth on WHO curves for weight, length and head circumference. Data showed similar growth patterns and safety profiles between the two infant formula groups over three and half months. No significant differences in adverse events were observed among the three groups.
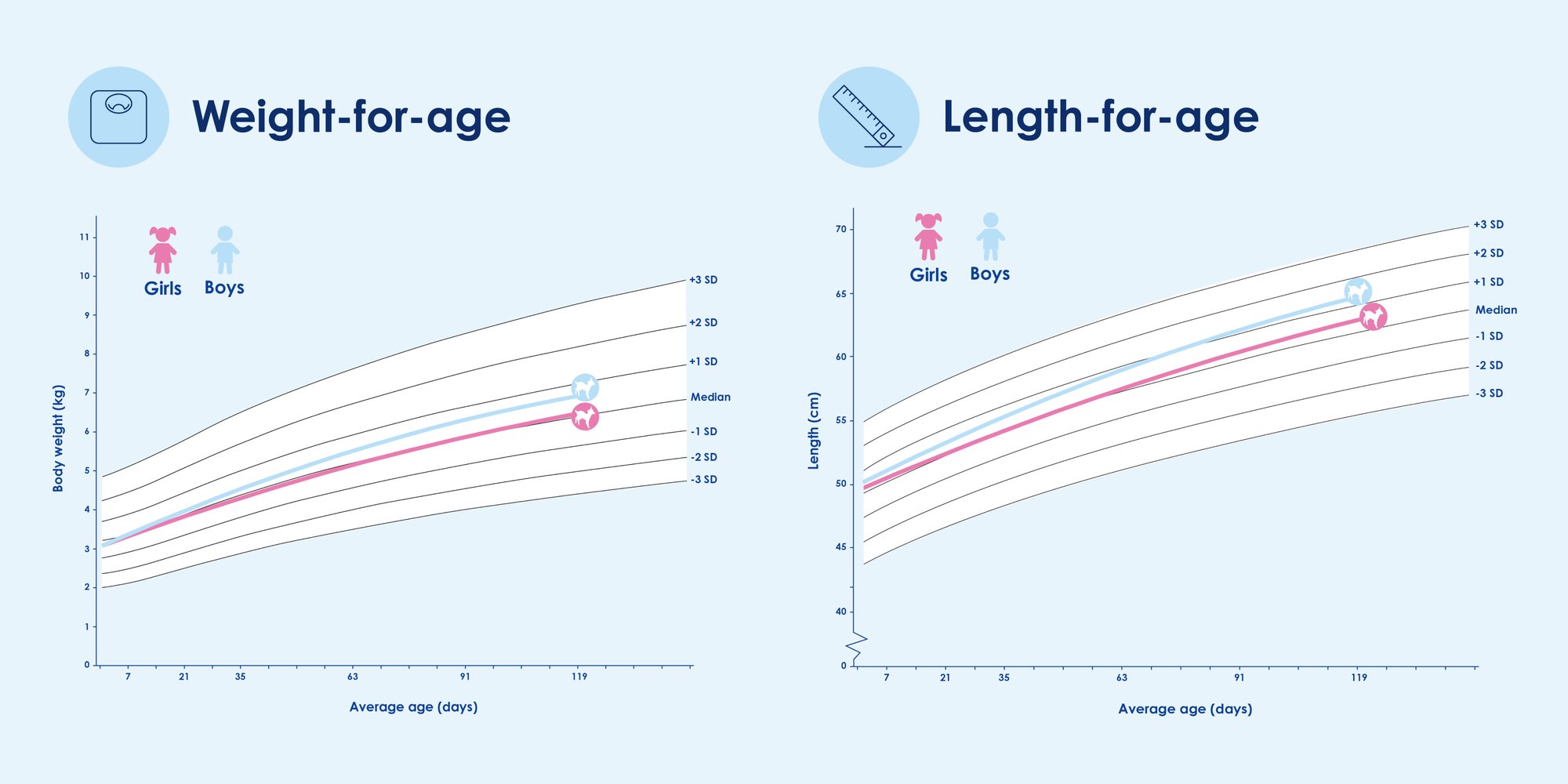
Conclusion
Kabrita Goat Milk-Based Infant Formula supports healthy growth, is well tolerated and is safe to use in infants from birth onwards, suggesting that Kabrita Goat Milk-Based Infant Formula is a viable option supporting adequate growth with good tolerability for infants.







